Riding on the back of a strong market, the TenStocks portfolio racked up decent year-to-date gains coming into late April. As qualifying investments became harder to find, I found myself over-weighting the few investments I thought still had good risk/reward characteristics.
Putting fewer eggs into a single basket increases the potential for both profits and losses. Unfortunately, we managed to find more of the latter. Our ill-fated investment in Aveo (AVEO) was sized so that in the worst case— an FDA rejection— our estimated loss would be limited to approximately our year-to-date gain.
I have previously published two articles about Aveo and its kidney cancer drug Tivozanib. This article will make more sense if you read those articles first. You can find the first here and the second here.
I have been working on the Aveo story for over a year. I was convinced the FDA would interpret the data results the same way I did and draw the same conclusions. They did not. I have no problem admitting that I was shocked and stunned at the result. We still are.
In early June, I was contacted by Mr. Robert Weisman of the Boston Globe. He’d read my articles on the Aveo story and wanted to dig deeper. I told him that I felt as though I was missing something; there was something I didn’t understand or didn’t know that would explain, or help to explain, why Tivozanib is now probably the best cancer drug the FDA won’t let cancer patients have.
The atmosphere at ODAC was so toxic from the FDA, I don’t understand how Aveo could have been so confident of approval. They even arranged Volvos for the sales reps! It looked as though the FDA went into the ODAC meeting thinking we’re going to crucify these guys, and Aveo went in thinking they’re going to give us hugs and kisses.
There was an obvious mismatch in expectation. If there were communication with the FDA prior to Tivozanib’s rejection, Aveo must have been tone deaf and must have drunk even more Kool-Aid than I did to miss the negative signals. It just seemed to me that a piece of the Aveo jigsaw puzzle was missing.
In early June, Aveo held a conference call to discuss the FDA’s rejection of Tivozanib. Some of the statements made during this call were shocking. Mr. William Slichenmyer, CMO of Aveo, stated the FDA had informed Aveo in May of 2012 that the overall survival data ‘will be a review issue that could affect approvability.’
In my view, this was material information that should have been fully disclosed to investors. Instead, Aveo announced the FDA had only expressed ‘concern’ about the overall survival (OS) data. Mr. Slichenmyer went on to say “we also discussed with the FDA about various designs for an additional trial.”
And David Johnson, Aveo’s CFO said “we have, for a period of months, starting from the pre-NDA meeting up until the ODAC meeting, had intermittent communications with the agency about study design.” And then, “the agency provided feedback to us with suggestions for different designs and we have subsequently gone back and forth with them on a couple of different alternatives, and not yet reached an agreement on what that study might be.” Mr. Slichenmyer continued, “some of the advice that we got along the way was that if there’s an ongoing next study, it makes it easy for the panel members to vote to simply wait for more data.”
Were Aveo deliberately foot-dragging to get to ODAC without doing the FDA-recommended additional OS trial? Why did they never disclose to shareholders that the FDA had informed them the OS data issue ‘could affect approvability’? Why did Aveo not disclose that they were in ‘intermittent communications’ with the FDA about the design and format of an additional OS trial right up to the May 2013 ODAC meeting?
It appears Aveo knew the OS data was a bigger issue for the FDA than what shareholders were told. It also appears Aveo knew the possibility (or probability) of the FDA requiring a new OS trial was greater than they led shareholders to believe.
If the information disclosed (for the first time) in this June conference call had been made public prior to Aveo’s fateful ODAC meeting, investors who lost money in Aveo may have decided to reduce or limit the size of their investment, or, they may have decided not to invest in Aveo at all.
In any event, in my view, there are questions about who knew what when, and about Aveo’s public disclosure obligations.
In early July 2013, the United States Securities and Exchange Commission (SEC) also began to take an interest in the Aveo story.
They sent Aveo a subpoena requesting documents and information concerning Tivozanib and related communications between Aveo, the FDA, investors and others. Meanwhile, of course, veritable busloads of lawyers piled on with class action lawsuits.
I could fill a hundred pages with what went wrong with Aveo. The bottom line is I was convinced the FDA would approve Tivozanib and I was wrong. The ODAC vote was 13-1 against. It wasn’t even close.
Here’s a simple chart:
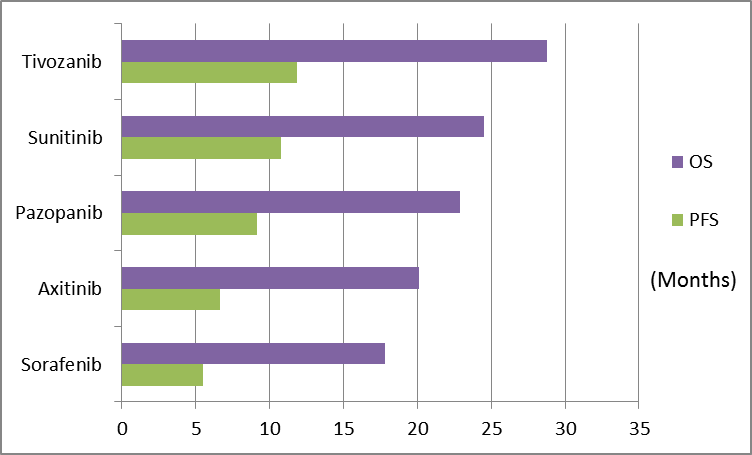
Source: FDA/ODAC briefing document (Table 2)
PFS is the primary endpoint for approval; OS is the secondary endpoint. All the renal cell cancer drugs in the above chart were approved by the FDA based on the PFS/OS statistics shown. Aveo’s Tivozanib was not approved based on the above statistics because, in the comparator arm of Tivozanib’s phase 3 Tivo-1 study, over 60% of those who took Sorafenib (the comparator) also took Tivozanib.
The FDA argued the hypothesis that Tivozanib contributed to superior OS performance (29.3 months versus 28.8 months) in the Sorafenib arm was not proven, and that the data, in their view, was ‘uninterpretable and inconclusive’, and they want another trial. For those interested, here’s the official ODAC meeting transcript.
I’ve done an enormous amount of work on this failed investment and that includes a rigorous post-damage autopsy. Going through all the data, and seeing all the FDA’s concerns and documentation, I just can’t find a way to understand, and agree with, the FDA’s stance.
In my opinion, Tivozanib is clearly superior to Sorafenib, an already approved drug, in both PFS and OS. Even a cursory examination of Sorafenib’s previous OS and PFS trial results would confirm that. However, it appears the FDA chose not to do that.
It’s my opinion the FDA reached their decision to reject Tivozanib without taking into consideration all available trial data. Even worse, the FDA may have used incorrect data to make their case against Tivozanib. In Aveo’s briefing document submitted to the FDA, on page 60, in Table 11, Tivozanib’s MSKCC prognostic groupings are shown as: Tivozanib: Favorable 26.9%, Intermediate 66.5%, Poor 6.5%
And in Aveo’s presentation for ODAC, on C-27, MSKCC prognostic groupings are the same but with rounded numbers. However, in the FDA’s ODAC presentation, on slide 12, the MSKCC groupings had changed to something very different: Tivozanib: Favorable 54%, Intermediate 45%, Poor 0.8%.
The FDA was telling the ODAC panel Tivozanib’s performance was suspect because the patients on the drug were not very sick. On page 7 of the FDA’s briefing document, the ODAC panel were told, “Note that a substantial number of patients had a MSKCC favorable prognosis.”
And then, as recorded on page 73, of the FDA/ODAC official transcript, Jonathon Jared, the FDA presenter at ODAC, while displaying slide 12 for the panel, said, “I show this information just to emphasize that the vast majority of the patients in this trial had favorable prognostic characteristics, and this impacts comparison of the results of this trial to that of others performed in the past.” Is this why the FDA did not take into consideration evidence of Tivozanib’s efficacy relative to other approved kidney cancer drugs as indicated in the chart above? Was it because they used incorrect data? Was this the prime source, or one of the prime sources, of the FDA’s negativity toward Tivozanib?
And perhaps a more important question, if the FDA did indeed use inaccurate data as part of its rejection of Tivozanib, is did Tivozanib get a fair trial?
The FDA’s job as public servants is not only to stop unsafe and ineffective drugs from getting to patients, but also to make sure the FDA themselves do not prevent a safe and effective drug from getting to patients. This is especially the case when it comes to cancer. Did Aveo run a perfect trial, cross all the T’s, jump flawlessly through all the FDA hoops in the required fashion?
Obviously not, but condemning a good drug to the trash heap by not considering all available data, by the possible misuse of incorrect data, and because of issues unrelated to the drug’s efficacy and safety does not serve anyone well.
For us, the FDA’s decision defies logic. It’s a complicated issue and we can’t lay it all out here, but we think the FDA has made a mistake. That may seem a presumptuous call for us to make, and maybe it is—after all, a truckload of FDA analysts and a panel of top cancer specialists emphatically disagreed with us. But there are a few kidney cancer specialists who seem to agree with us. One of them is Dr. Thomas Hutson of Baylor Charles A. Sammons Cancer Center in Dallas.
Here’s a recent video of him talking about the FDA’s decision. Another is Dr. Robert Motzer of Memorial Sloan-Kettering Cancer Center in New York. Here’s a video of him talking about the Tivozanib OS data, and here is another where he talks about the efficacy and safety of Tivozanib. We should point out that both these kidney cancer experts have been financially compensated by Aveo (which is more than we can say).
Anyway, the damage to the portfolio is done. I will take the hit and move on. So what do I do with Aveo now, and where do I go from here?
While Aveo has recently said it will not run another OS trial for kidney cancer, Tivozanib trials are still on-going for breast and colon cancer. It’s a good drug; I think it’s the best drug in renal cell cancer based on the data, and it still has value.
I think Aveo’s best options now are to sell the company to a bigger pharmaceutical company, or to sell Tivozanib to a bigger pharmaceutical company that can finance the needed trials and are better equipped to bring the drug through the regulatory process. I think a buyout or restructure deal should come sooner, rather than later. Aveo is burning cash and needs to move fast.
Pharmaceutical companies almost never visit my website at tenstocks.com. Most of my visitors are individual investors, hedge funds and investment banks. Interestingly, and make of it what you will, in the last six weeks, we’ve had visitors from Merck, Sanofi-Aventis and Baxter Healthcare.
Aveo shares currently sell for around the value of its cash-on-hand at about $2.50. Throw in something for a good cancer drug they can’t sell, add a bit more for the pipeline, which includes the RON receptor antibodies deal with Centocor, now a part of Johnson and Johnson (Aveo is eligible to receive up to $540 million in milestone payments), toss in a few pennies for Aveo’s Human Response Platform, and in a buyout scenario right now, I think Aveo could be worth $5 to $6 a share. If there’s no deal, current Aveo shareholders face a long, hard road as cash depletes.
One irony for me with the Aveo/Tivozanib saga is that one of the reasons I decided to write and publish our Aveo articles on Covestor and, for the first time, on Seeking Alpha, was to let those interested in my work see how deep I dig to maximize the probability I will get our investment decisions right. I never expected that perhaps the best example of my work would be an investment we got wrong.
The investments discussed are held in client accounts as of July 31, 2013. These investments may or may not be currently held in client accounts. The reader should not assume that any investments identified were or will be profitable or that any investment recommendations or investment decisions we make in the future will be profitable. Certain information contained in this presentation is based upon forward-looking statements, information and opinions, including descriptions of anticipated market changes and expectations of future activity. The manager believes that such statements, information and opinions are based upon reasonable estimates and assumptions. However, forward-looking statements, information and opinions are inherently uncertain and actual events or results may differ materially from those reflected in the forward-looking statements. Therefore, undue reliance should not be placed on such forward-looking statements, information and opinions.


