When the FDA got together with the Oncologic Drugs Advisory Committee (ODAC) in early May to discuss whether to approve Aveo Pharmaceutical’s (AVEO) Tivozanib for the treatment of renal cell carcinoma (RCC), it would be fair to say the FDA reception was not what AVEO was expecting.
After all, they were so sure of approval they had interviewed and hired sales staff and planned launch details, even down to deciding upon the kind of cars their sales reps would be driving. Instead, the FDA was merciless, ripping apart Aveo, Tivozanib, the phase 3 trial design and data, and just about everything and everyone involved in trying to bring this drug to market. People who witnessed the meeting described it as ‘brutal’ and ‘scathing.’ After a morning of spirit-wilting torture, and just a few minutes before the scheduled lunch break, the ODAC committee was asked to vote on a carefully worded question:
“Has the Applicant demonstrated a favorable benefit-to-risk evaluation for the treatment of renal cell carcinoma in an adequate and well-controlled trial?” The panel voted 13-1 that the answer to this question was no.
The show was over. The good guys had won; the guardians of our public health had done their job. Those suffering with terminal RCC would be protected from the potential dangers and ineffectiveness of Tivozanib. Maybe the FDA will give Tivozanib another chance if Aveo does a new trial and manages to come up with a better and more acceptable set of benefit-to-risk data. A new trial would likely take at least three years. In this scenario, Tivozanib would not be available until 2017. Between now and then, approximately 56,000 people will die from RCC in the U.S. alone.
To better understand what went wrong, we need to examine the two main components of FDA approval. These are progression-free survival (PFS) and overall survival (OS). For many years, the FDA has insisted PFS is the primary endpoint as it is considered to be more reliable and measurable, while OS is often harder to measure and often confounded by issues of crossover and the use of sequential therapy. For now, let’s take the FDA at their word and explore PFS.
Progression- free survival (PFS) is the length of time a disease does not get worse (progress) while someone is taking, and after they have taken a drug. Let’s take a look at the following PFS chart.
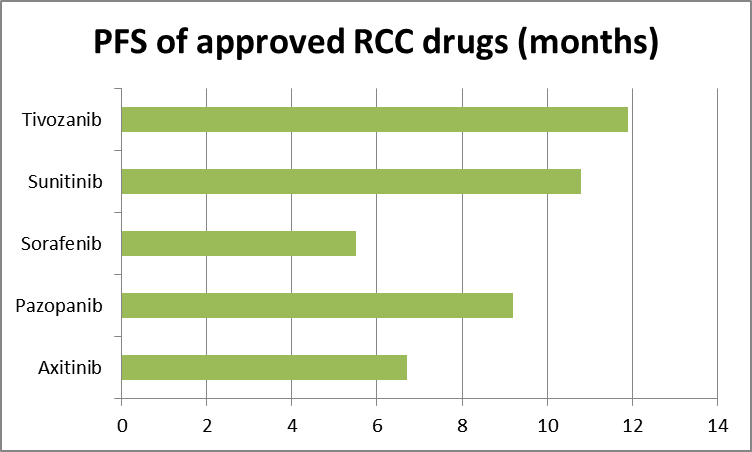
Source: Tenstocks.com, FDA/ODAC briefing document table 2.
All the drugs in the above chart were approved by the FDA based on the PFS shown. Tivozanib has not been approved. If the primary endpoint for approval was PFS, and the Tivo-1 phase 3 trial was designed to measure PFS, why won’t the FDA approve Tivozanib when they approved all the others? After all, by using the FDA’s own data, Tivozanib appears to be best in its class.
The use of RCC drugs in the first-line setting can offer cleaner data because the patients are treatment naïve. If Tivozanib is indeed the best in class, its supremacy should show up here too. The current accepted standard of care in RCC is Sunitinib—marketed by Pfizer (PFE) as Sutent—and Pazopanib —marketed by GlaxoSmithKline (GSK) as Votrient. They are considered the best available drugs for RCC. A look at treatment-naive data from various previous trials shows the same pattern of Tivozanib outperformance.
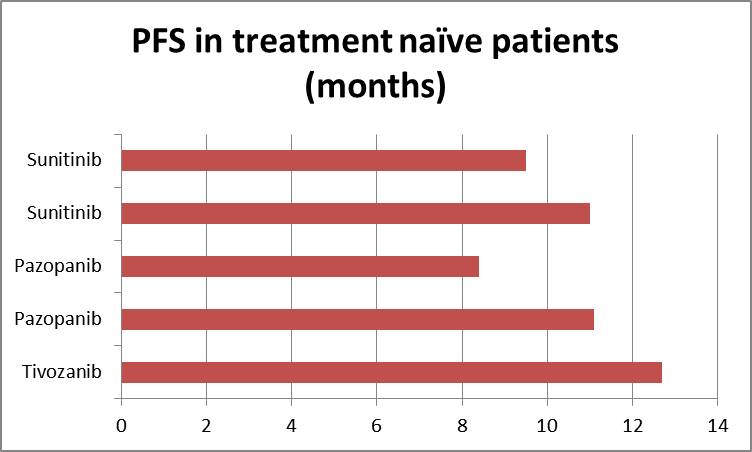
Source: Tenstocks.com, Aveo slide 5
There seems little doubt that when it comes to PFS, Tivozanib is the best in class. If the other drugs were approved based on PFS, Tivozanib clearly should be approved as well.
However, the FDA is now saying they have not changed the rules for approval and that PFS is still the primary endpoint, but OS is now the gold standard of endpoints. If you find this slightly confusing, join the club.
So, we move on to OS, the FDA’s gold standard. Take a look at this OS chart:
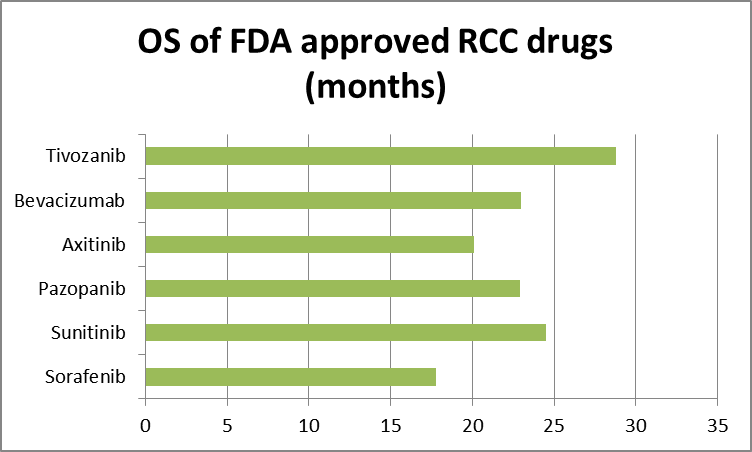
Source: Tenstocks.com, FDA briefing document table 2.
Here again, we see an indicator of Tivozanib’s supremacy. Again, realize all the drugs in the above chart have been approved based on the data shown—all except Tivozanib. This doesn’t make logical, rational sense, does it? After all, PFS is clearly a predictor of OS, and OS is clearly a predictor of PFS. If a drug outperforms in both endpoints—primary and gold, as the FDA calls them—its performance confirms superiority over all other approved RCC drugs and it also confirms efficacy and safety.
The FDA does not think Tivozanib has demonstrated a favorable benefit-to-risk in an adequate and well-controlled trial. The biggest issue for the FDA was the OS result in Aveo’s phase 3 Tivo-1 trial. The OS for Tivozanib came in at 28.8 months, as shown in the OS chart above. The problem was the Sorafenib comparator arm came in at 29.3 months. Sorafenib—marketed by Bayer (BAYRY) and Onyx Pharmaceuticals (ONXX) as Nexavar— outperformed Tivozanib.
This resulted in a hazard ratio (HR) of 1.25. The FDA has never approved a drug for RCC with an HR greater than 1. The difference between the two arms—28.8 versus 29.3—was not statistically significant, but the FDA decided the HR was. So, at Aveo’s ODAC meeting, the FDA introduced a new endpoint for approval: The HR had to be less than 1.
When looking at the issue of HR in this case, it’s important to note 61% of the patients who started on Sorafenib crossed over to Tivozanib upon progression. This meant the trial involved two active drugs against one. It was Sorafenib/Tivozanib versus Tivozanib alone.
Now, Tivozanib achieved PFS of 11.9 in this trial. Sorafenib reached a PFS of 9.1. However, in the Sorafenib arm, the 61% who crossed over and had sequential therapy on Tivozanib achieved an additional PFS of 8.4 months. If the 61% who crossed over in the comparator arm had a median PFS of 9.1 while on Sorafenib, then they had a total PFS of 17.5 months when combined with Tivozanib. Is it any wonder OS in the comparator arm was higher?
When it comes to approval, The FDA now states an HR of less than 1 favors the investigational drug and an HR greater than 1 does not. Here we have an HR of 1.25. Pazopanib was approved with an HR of .91 but it was compared to placebo and 54% crossed over to Pazopanib. So the comparison was 100% Pazopanib versus 54% Pazopanib. Sorafenib was also approved based on an OS of 17.8 months with an HR of .88 against placebo. Getting an HR of less than 1 is easy when you compare your drug to a placebo or a half dose of the same drug. Tivozanib was compared to an approved and effective drug and to Tivozanib.
Other RCC drugs, including standard of care Sunitinib, were approved based on comparison to IFNa, a minimally effective drug. Again an easy HR bar to clear.
Of all the drugs approved for RCC, only Axitinib—marketed by Pfizer (PFE) as Inlyta— was compared to a really active and approved RCC drug. Again, Sorafenib was used as the comparator. The HR came in at .97 but no crossover was allowed, so the comparison was straight Axitinib versus Sorafenib.
How would OS for Tivozanib versus Sorafenib look with no crossover? Look at this chart:
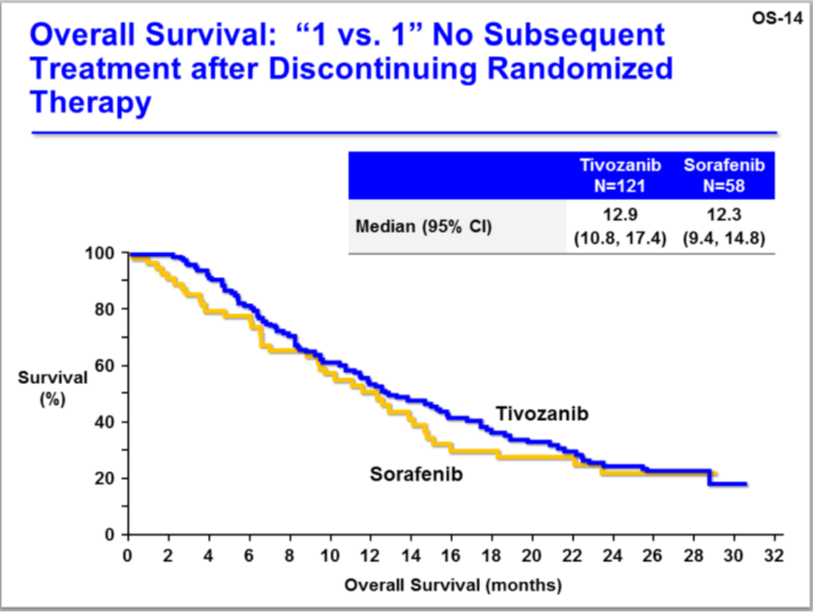
Source: FDA, Aveo slide OS-14
The OS trend favors Tivozanib.
If Aveo’s RCC drug was negatively impacting OS, it would also show up in serum levels. Analysis of the OS data and serum levels show no indication of a higher incidence of death with higher serum levels of Tivozanib. Indeed, the most likely cause of death due to Tivozanib would likely come from hypertension, which is Tivozanib’s main side effect.
Its incidence is higher with Tivozanib than with all other RCC drugs. But hypertension is also a marker of efficacy in RCC. A subgroup with hypertension in the Tivo-1 trial reached a PFS 18.3 months. This is yet another indicator of Tivozanib being the best-in-class RCC drug.
Essentially and importantly, Tivozanib is the first RCC drug to be compared to not one, but two active drugs, and its use, when combined with Sorafenib, achieved an OS of 29.3 months, the highest ever recorded in an RCC trial, and 28.8 months when used alone. Results such as this would just not be possible if Tivozanib was inferior to the other already approved drugs. In the case of Tivozanib, an HR of 1.25 demonstrates the effectiveness, not inferiority, of Aveo’s cancer drug. Nevertheless, the FDA is insisting on using a comparative benchmark of HR=<1 as if Tivozanib had been compared to a placebo or IFNa.
In my opinion, the error Aveo made in the Tivo-1 trial was not doing a two-way crossover. Patients on both arms should have been able to cross over to the other drug upon progression. This would have balanced out the effects of two drugs versus one and the HR would almost certainly have been below 1.
In RCC, cancer drugs are used in sequence. When one drug ceases to work, patients are switched to another drug until progression and so on. Drug side effects also play a role. All RCC drugs are toxic and cause a wide range of adverse events. Some are better tolerated than others. Dosage often has to be reduced, interrupted or discontinued altogether, depending on the patient’s ability to tolerate a particular drug. This is why having a wide range of options in the treatment of RCC is important. For a patient suffering with RCC, the more options the better.
At Aveo’s FDA ODAC meeting, the single vote for approval of Tivozanib was from a patient advocate. Members of the public were also allowed to address the ODAC panel. One by one they offered heartbreaking testimony to the efficacy of and need for Tivozanib. They were doctors and nurses, caregivers and patients. Each had personal experience of RCC and some of them had been taking Tivozanib in trials. Of the nine people who spoke, eight of them pleaded for the approval of Tivozanib (the ninth suggested another trial may be needed).
According to an article in the Wall Street Journal, the FDA’s cancer czar is Dr. Richard Pazdur. In the article, the co-founder of a group that wants patients to have wider access to cancer drugs calls Dr. Pazdur “a lethal disaster for cancer patients.” Indeed, it was Dr. Pazdur who led the blistering attack on Aveo and Tivozanib at the FDA’s ODAC meeting. He is quoted by the Wall Street Journal in a related interview as saying, “the major issues with the oncology drugs as mentioned before are efficacy, demonstration of efficacy, kind of like in the real-estate industry the mantra being location, location, location, in oncology it’s efficacy, efficacy, efficacy.”
He went on to say that “the reason why oncology drugs don’t get approved is basically because they fail to demonstrate efficacy… A lot of the misconceptions people have revolve around their own misunderstanding of the field of statistics and whether or not a trial meets its primary endpoint and why that is important.”
Okay. We’re back to the primary endpoint. That’s what is important. Tivozanib met its primary endpoint. So it should be approved, right? Dr. Pazdur again: “one does not want to see a potentially active drug not be available to the American public.”
Neither do cancer patients, or their families and friends. But if the FDA doesn’t approve Tivozanib, isn’t that exactly what’s happening here?
At Aveo’s ODAC meeting, Dr. Pazdur, expressing his concern about Tivozanib said, “obviously overall survival is a much more important clinical endpoint than progression-free survival.”
Yet, while at the ODAC meeting to discuss Axitinib, which was approved, he said “to say that we have to demonstrate an overall survival advantage from the last approved drug for any other new drug to be approved is a very high bar here and really is a comparative efficacy standard, which we do not have the legal authority to impose.”
And then he said, “we have to be consistent with other sponsors, and we have a track record here that has ranged of six drugs that have been approved and six companies given advice with a PFS endpoint, so we can’t hold one sponsor up to a higher standard than another sponsor; that’s for sure.”
So we go round and round on the FDA merry-go-round about what the approvable endpoint is and what it isn’t. Meanwhile, there will be about 65,000 new cases of RCC this year in the US alone. About 14,000 Americans will die of RCC this year. In the Tivo-1 trial, after Sorafenib stopped working, Tivozanib halted disease progression for 8.4 months.
When the FDA is making life and death decisions for cancer patients, it’s not the FDA or Dr. Pazdur that does the dying. If the FDA is so concerned about the Tivozanib OS data, a pragmatic, logical, sane and compassionate solution for all sides would be to approve Tivozanib for patients suffering with RCC and order post-approval monitoring of OS.
Tivozanib is also being studied for efficacy in liver, breast, colorectal and other cancers. Those in the cancer community who want to see this effective drug approved need to speak up now. When it comes to cancer, the dead don’t talk. For cancer patients, that’s the real endpoint.
The investments discussed are held in client accounts as of May 31, 2013. These investments may or may not be currently held in client accounts. The reader should not assume that any investments identified were or will be profitable or that any investment recommendations or investment decisions we make in the future will be profitable.


